CSF Management - HP Bio
CSF Management inc EVD, Shunts & Catheters
Delta have partnered with HPBio (HP Biopróteses Ltda), a Brazilian medical device company specialising in the development and manufacture of neurosurgical implants, particularly hydrocephalus shunt systems. Established more than 25 years ago, the company is known for its Sphera® line, which includes the Sphera, Sphera Duo & Sphera Pro. HPBio emphasises biocompatibility and durability in its products, using materials such as medical-grade silicone, polysulfone, titanium, and ruby components. Their shunts are designed for use across adult, pediatric, and neonatal patients, with innovations like the MRI-safe programmable Sphera Pro valve (compatible with 3T MRI) gaining international recognition.
To speak to one of our clinical product specialists or to order accessories and consumables please call 01782 637009. Alternatively send an email by clicking here and we will contact you within 24hrs.

SPHERA ANTI-SIPHON CEREBRAL SHUNT SYSTEM
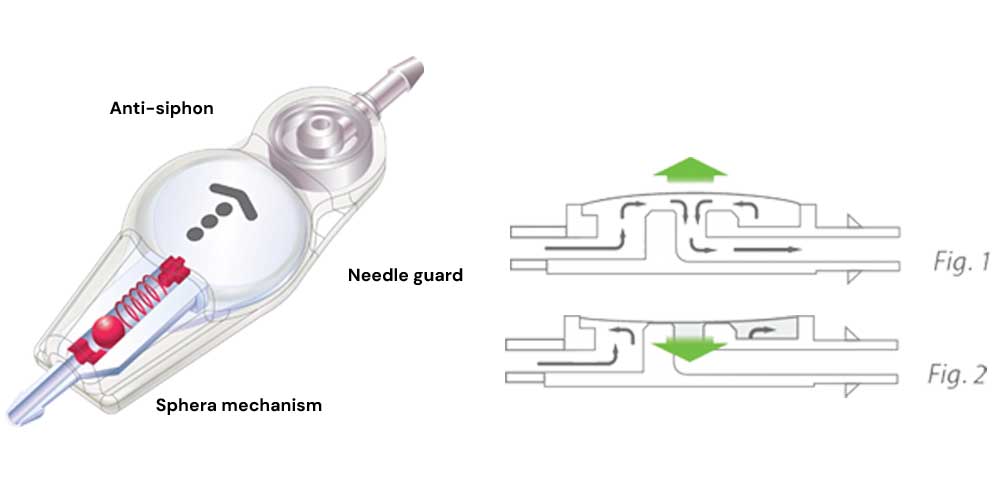
The Sphera Valve with Anti-siphon is designed to maintain the Cerebral Intraventricular pressure stable regardless the position of the patient, reducing the occurrence of overdrainage. The valve is flexible, with anatomic design and low implant profile. It is made in transparent medical grade silicone with internal structure in polysulone. It has a central pumping chamber with needle guard to protect against excessive penetration during puncture.
Encased in the output connector, the mechanism prevents ventricular overdrainage caused by siphoning in the distal catheter when the patient moves from horizontal to vertical position.
The mechanism is composed of a flexible silicone membrane that when attracted by the negative pressure of the distal catheter prevents or reduces the flow of excessive fluid (fig.2). When the patient is upright, the combination of the mechanisms Anti-siphon and Sphera provides the dynamic balance of the system, keeping the valve operating on a stable
flow/pressure rate.
When the patient returns to the horizontal position, the Anti-siphon system stops to interfere in the control of flow and the valve returns to work in the initial condition (Fig.1).
HYDROCEPHALUS SHUNT SYSTEM SPHERA DUO
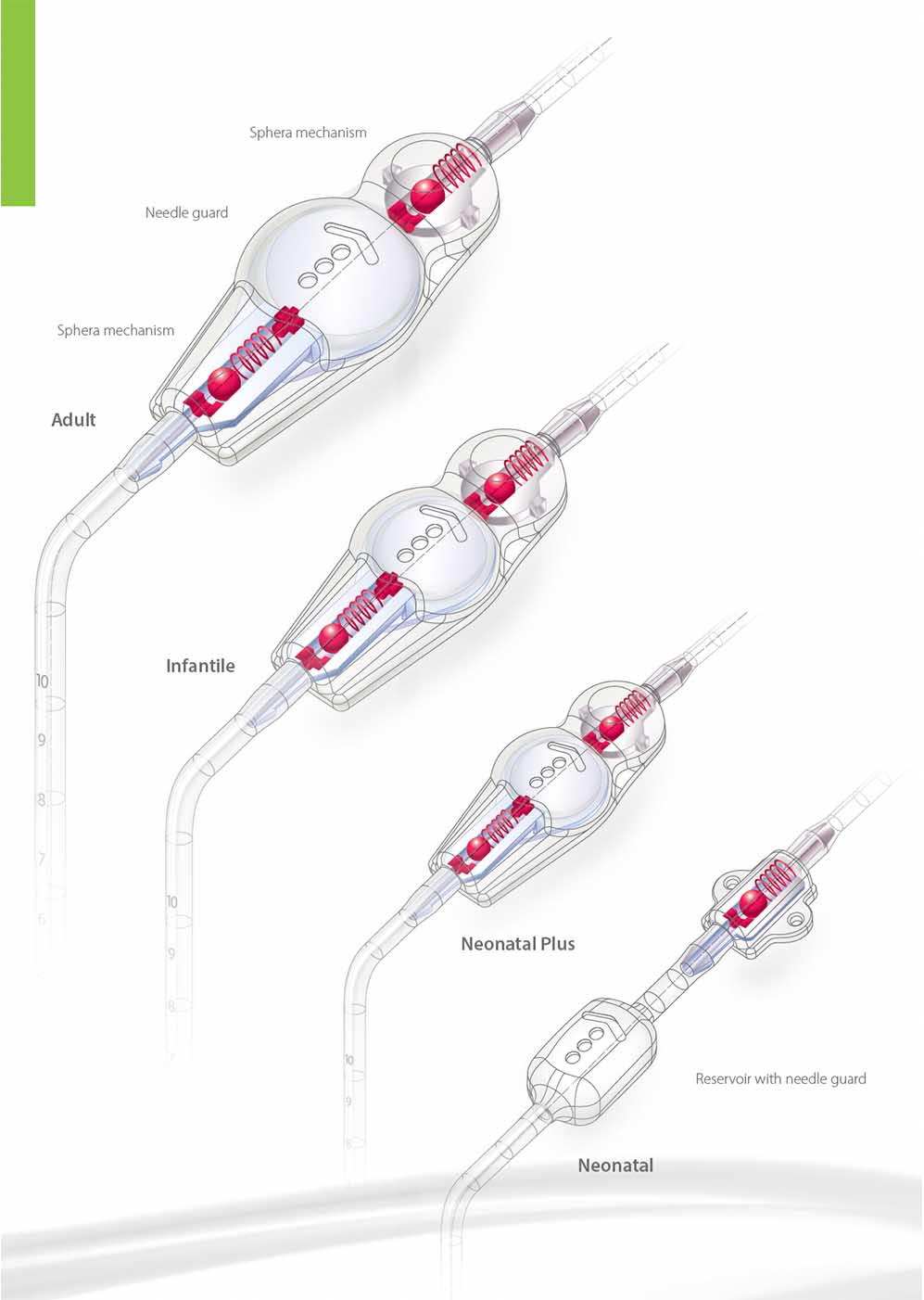
Sphera Duo valves were developed for a precise control of cerebral intraventricular pressure. The Adult, Infantile and Neonatal Plus sizes design present low profile of implant and flexible body with anatomical shape to contour the curvature of the cranium.
The valves are made of transparent medical grade silicone with internal structure in polysulfone. They have pumpable central reservoir with needle guard that protects against perforations on punctioning or sampling procedures. The Neonatal size has no pumping chamber, as the design prioritises low profile and minimum implant volume. The system can be combined to a reservoir connected to the ventricular catheter to enable puncturing and CSF sampling.
SPHERA MECHANISM: PRECISE MANAGEMENT
The pressure control system is composed of ruby ball and conic seat and stainless steel spring. The perfect match between ball and seat can safely set the pressures of opening and closing of the system, providing precise control of intracranial pressure.
Adult, Infantile and Neonatal Plus sizes have a dual pressure control encased in the input and output occluders. The Neonatal model encases a single mechanism for pressure control.
All sizes are supplied in four pressure ranges: high, medium, low and extra low, to meet the individual requirements of patients. The indications of flow and pressure printed on the valve body are radiopaque and allow visualization imaging after implantation.
CATHETER: FLEXIBILITY AND RADIOPACITY
The shunt system presents the valve accompanied by cerebral ventricular catheter and peritoneal catheter. These are made of soft transparent medical grade silicone with radiopaque stripe, which ensures catheter visualization in imaging exams. The hardness of the silicone used in the manufacture of catheters was tailored to allow adequate flexibility and at the same time, prevent the occurrence of unwanted kink in the subcutaneous route, which can cause obstruction or decrease the flow of drainage. For all valve sizes, the system can be supplied in different configurations of peritoneal and ventricular catheter with or without reservoir.
SPHERA PRO - HYDROCEPHALUS PROGRAMMABLE SHUNT
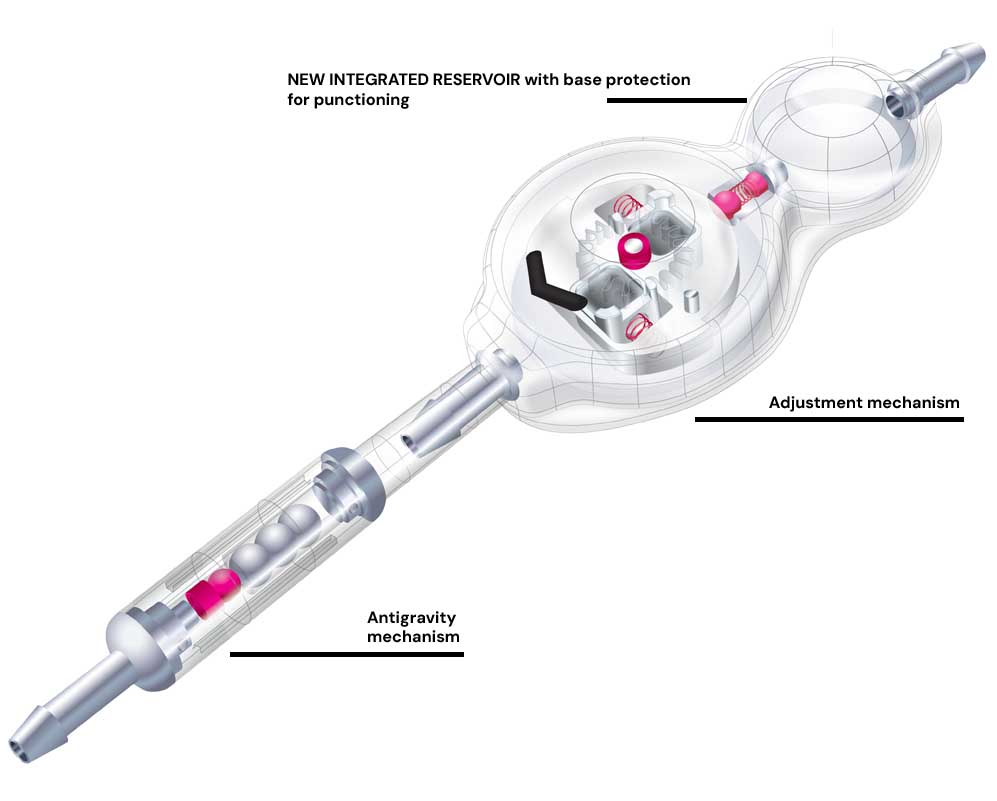
Sphera Pro is a cerebral shunt system for intraventricular pressure control with a programmable valve. This product has been developed to provide ACCURACY on pressure control, SECURITY against deprogramming and EASY
ADJUSTMENT procedure.
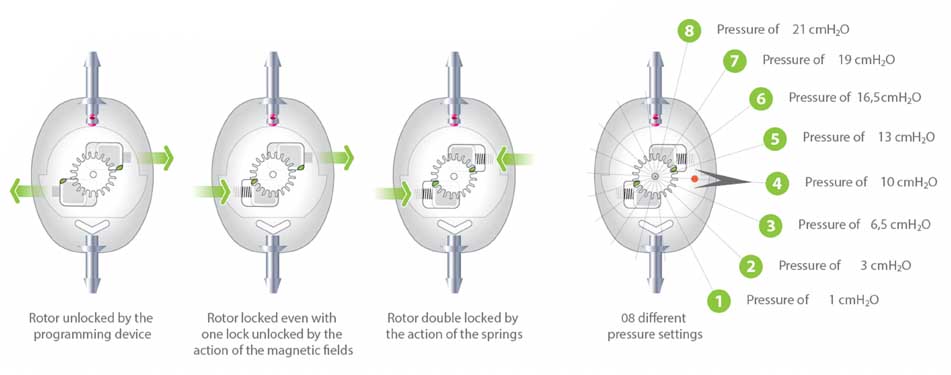
The valve has 8 different pressure settings, and pressure adjustment can be performed whenever necessary by a non-invasive device and painless to patients.
Safety against magnetic fields deprogramming is provided by an exclusive double locking system, which holds the valve at the chosen pressure even when the patient undergoes MR examinations of up to 3 teslas.
The Sphera Pro system can be supplied along with the Sphera Grav antigravity device which acts in intraventricular pressure control when the patient changes from the horizontal to the vertical position, preventing overdrainage especially for patients with Normal Pressure Hydrocephalus (NPH).
The valve and reservoir are made of polysulfone with a silicone coating and titanium connectors. The system’s ventricular and peritoneal catheter are made of transparent silicone with a radiopaque fillet which enables the visualisation in imaging tests and minimizes the occurrence of calcification in its path.
ACCURACY WITH THE ADJUSTMENT MECHANISM
During the adjustment, the rotor movement changes the distance between the rotor axis and the Sphera mechanism (spring, sphera and conical ruby seat), increasing or decreasing the valve pressure. The precise rotor radius dimensional control allows the accurate definition of the 8 different levels of opening and closing pressures, providing a real intracranial pressure control when the pressure adjustment is performed.
SECURITY AGAINST DEPROGRAMMING
With a unique design, the valve rotor has two mechanical safety locks that moves in opposite directions, and may have double or single locking against non-intentional deprogramming. Each lock is located on a magnet and is pushed against a pressure position by the action of a spring.
When the magnetic fields from the environment, or an unilateral one (generated by MRI), are able to move one of the magnets and release one of the latches, the other is forced by the same fields to remain in the locking position, avoiding deprogramming of the valve.
EASY PRESSURE ADJUSTMENT
The programming device allows an easy pressure adjustment as it provides easy valve location, painless to the patient and immediate confirmation of the adjusted pressure, eliminating the necessity of image exams.
Since the correct programming device centralization upon the valve is essential to the unlocking and pressure adjustment, the reader has an indication alignment system that confirms the right position.
ANTIGRAVITY DEVICE - Hiperdrainage control
The antigravity device Sphera Grav provides overdrainage control by performing automatic compensation for the system’sopening pressure as the patient postural changes.
When the patient is in the supine position, no resistance will be built by Sphera Grav, only the Sphera Pro valve will be active. However, when the patient is in the orthostatic position, the maximum resistance of Sphera Grav will act along with the Sphera Pro adjustment pressure, making the shunt system more physiological and maintaining the intraventricular pressure more constant and with less risks of overdrainage. Sphera Grav has 06 resistance options (10, 15, 20, 25, 30 or 35 cmH2O) to meet the different needs of pressure control for each patient. The device consists of tungsten and ruby spheres, polysulphone body, titanium connectors and silicone coating.